I recall standing in the boardroom of a massive petrochemical refinery during a Stage 2 surveillance audit, watching the Operations Director argue with the EHS Manager. The facility was producing record-breaking output with minimal product defects, yet they were bleeding money through regulatory fines for improper wastewater disposal. The Operations Director held up a glowing Quality report, while the EHS Manager held up a "Non-Conformance" citation from the environmental agency. It was the perfect illustration of a disjointed organization—one that had mastered the art of making a product but had failed to manage the ecosystem in which it operated.
This disconnect is dangerous, expensive, and unfortunately common, but it is exactly what Quality Management Systems (QMS) and Environmental Management Systems (EMS) are designed to prevent. In this guide, I will break down exactly what these systems are, the global standards that govern them (ISO 9001 and ISO 14001), and why the most successful companies I audit don't treat them as separate silos. We will explore the definitions, the critical differences, and the immense value of integrating them into a unified framework that ensures both customer satisfaction and environmental stewardship.
Defining the Systems: QMS vs. EMS
To understand how these systems interact, we must first define their individual mandates. In my experience as an auditor, confusion often arises when organizations try to apply quality metrics to environmental issues or vice versa.
What is a Quality Management System (QMS)?
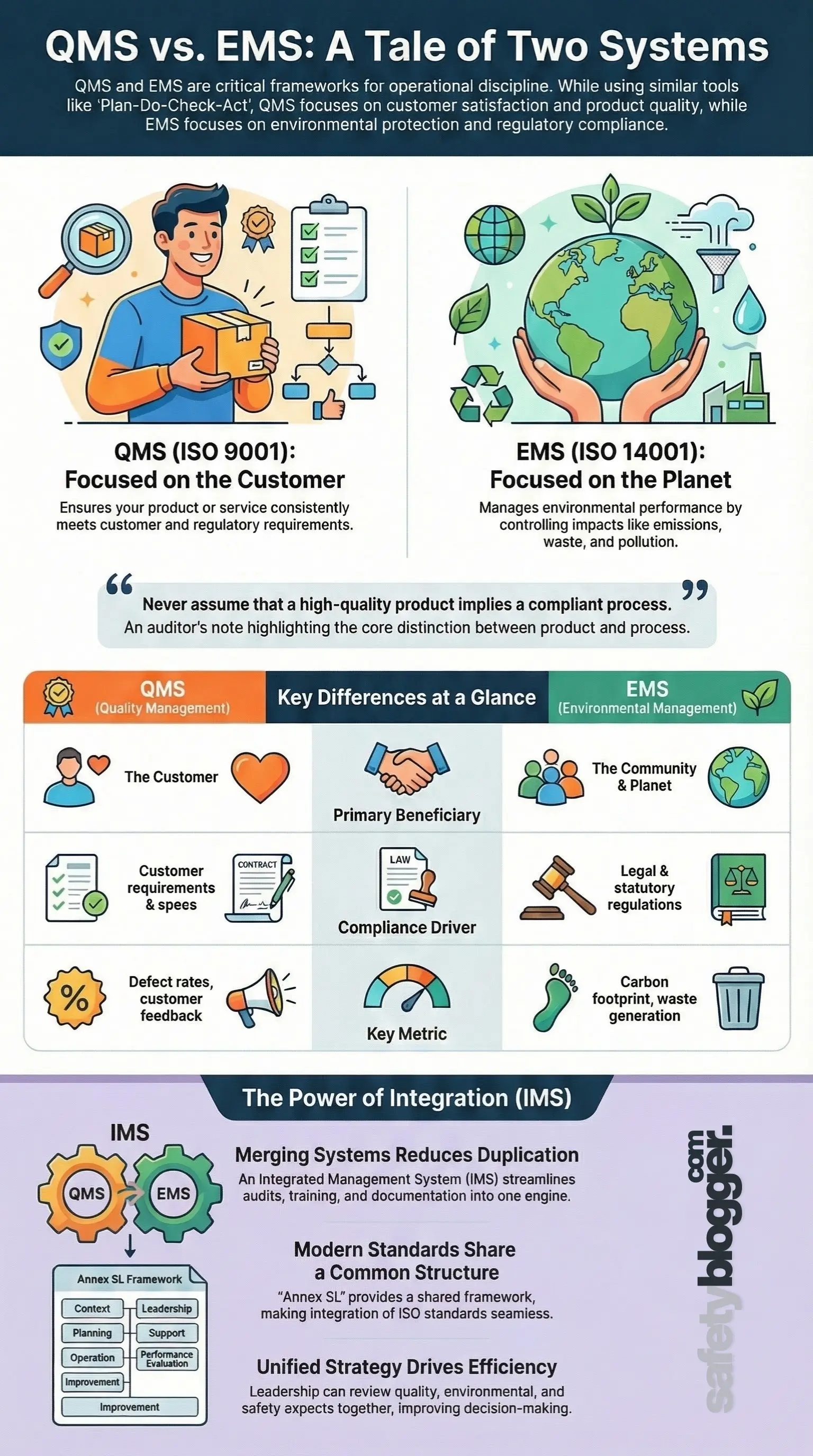
A Quality Management System (QMS) is a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives.1 Its primary focus is the customer. The goal is to ensure that your product or service meets specific requirements consistently.
The Gold Standard (ISO 9001): This is the globally recognized benchmark for QMS.2 When I audit against ISO 9001, I am looking for evidence that the company can consistently provide products that meet customer and regulatory requirements.
Key Elements: It involves rigorous document control, process mapping, and a heavy reliance on the "Plan-Do-Check-Act" (PDCA) cycle to fix defects before they reach the client.
What is an Environmental Management System (EMS)?
An Environmental Management System (EMS) is a framework that helps an organization achieve its environmental goals through consistent review, evaluation, and improvement of its environmental performance.3 While QMS looks outward to the customer, EMS looks outward to the planet and the community.
The Gold Standard (ISO 14001): This standard maps out a framework that a company or organization can follow to set up an effective EMS.4 It does not state specific environmental performance criteria but provides the map to meet them.
Key Elements: The core here is identifying "Aspects and Impacts"—knowing exactly how your activities interact with the environment (e.g., emissions, waste) and controlling them.
Key Differences Between QMS and EMS
In my experience auditing Integrated Management Systems (IMS) across petrochemical refineries and offshore platforms, I often see confusion where organizations try to force-fit environmental controls into quality procedures. While both systems share the "Plan-Do-Check-Act" (PDCA) cycle, their ultimate trajectories are fundamentally different: one looks inward at the product, and the other looks outward at the impact.
Below is a breakdown of the critical distinctions between a Quality Management System (QMS) and an Environmental Management System (EMS).
Feature | QMS (Quality Management System) | EMS (Environmental Management System) |
Primary Standard | ISO 9001:2015 | ISO 14001:2015 |
Core Focus | Product consistency and customer satisfaction. | Environmental protection and pollution prevention. |
Primary Beneficiary | The Customer (and the business bottom line). | The Community, Regulators, and the Planet. |
Scope of Control | Processes that affect product/service quality. | Processes that interact with the environment (Aspects/Impacts). |
Compliance Driver | Customer requirements and product specs. | Legal regulations and statutory requirements. |
Key Metric | Defect rates, customer feedback, yield. | Carbon footprint, waste generation, emissions. |
The Divergence in Focus and Scope
The most distinct difference I look for during an audit is the "Target of Care." In a QMS audit, I am scrutinizing whether a valve is manufactured to the exact engineering tolerances required by the client; if it holds pressure and doesn't break, it passes.
However, when I switch my hat to an EMS audit, I don't care if the valve works perfectly for the client. I care about the process of making that valve—did the cutting fluid leak into the groundwater? Was the scrap metal recycled properly?
QMS is Customer-Centric: It ensures the output meets the buyer's needs.
EMS is Stakeholder-Centric: It ensures the output doesn't harm the neighbors or the ecosystem.
Regulatory vs. Contractual Obligations
During my time working on large-scale construction projects in the Middle East, I noticed that QMS failures usually resulted in financial penalties or lost contracts. If the concrete mix wasn't right, we lost money.
EMS failures, on the other hand, resulted in legal action. Violating environmental regulations (like ISO 14001 mandates) carries the weight of federal law, fines, and potential shutdowns.
QMS: Driven by contracts, market demand, and ISO 9001 specifications.
EMS: Driven by local EPA laws, international protocols, and ISO 14001 compliance.
Auditor Note: "Never assume that a high-quality product implies a compliant process. I have seen world-class products manufactured in facilities that were actively leaching chemicals into the soil. As an auditor, you must separate the 'what' (product) from the 'how' (environmental impact)."
Integration in Modern Industries
Despite these differences, modern standards (Annex SL) have aligned the structures of ISO 9001 and ISO 14001 to make integration easier.
When I implement an IMS (Integrated Management System), I look for the overlap. For instance, reducing waste is a quality objective (efficiency) and an environmental objective (pollution prevention).
Shared Goal: Both systems aim for "Continual Improvement."
Shared Tool: Both utilize the PDCA cycle and Risk-Based Thinking.
Shared Burden: Both require document control, internal audits, and management reviews.
Pro Tip: If you are preparing for an external audit, do not present your QMS and EMS as entirely separate silos. Auditors like myself prefer to see an "Integrated Aspect-Impact Register" where you identify how a quality failure (e.g., a leaking tank) immediately becomes an environmental impact. This demonstrates OTHM-level strategic thinking.
The Power of Integration: Integrated Management Systems (IMS)
The most efficient organizations I work with do not run these systems in parallel; they merge them. An Integrated Management System (IMS) combines all related components of a business into one system for easier management and operations.
Breaking Down the Silos
The true power of integration lies in the removal of operational silos. When safety and quality compete for attention, safety often loses to production pressure, or quality suffers due to rigid safety constraints. An IMS harmonizes these priorities.
For example, when I audit a unified "Change Management" procedure in an IMS, I am verifying that a modification to a pressure vessel is checked for engineering integrity (Quality), leak potential (Environment), and explosion risk (Safety) simultaneously.
Unified Strategy: Management reviews cover all aspects at once, ensuring that business decisions consider safety risks and quality goals together.
Reduced Duplication: Instead of three separate internal audit schedules, training matrices, and document control systems, you have one streamlined engine.
Cost Efficiency: Third-party certification audits (External Audits) are significantly shorter and cheaper when auditing an IMS compared to three separate standards.
The Role of Annex SL
The reason modern integration is seamless is due to Annex SL (now ISO/IEC Directives, Part 1). This is the high-level structure that ISO applied to all recent management system standards.
Whether I am reading ISO 45001 or ISO 9001, Clauses 1 through 10 have the same titles and core requirements (e.g., Clause 4 is always "Context of the Organization," and Clause 9 is always "Performance Evaluation"). This structural alignment allows organizations to plug different standards into the same backbone without friction.
Auditor Note: "During an audit, I look for a 'Golden Thread'—a single policy that commits to safe working conditions, customer satisfaction, and environmental stewardship. If your policy is fragmented, your culture likely is too. An IMS is not just about combining manuals; it is about combining mindsets."
Real-World Application
In practice, integration transforms the daily life of a worker. Instead of filling out a Quality Checklist, a Safety JSA, and an Environmental Waste Log, the worker utilizes a single Operational Control Document.
I often tell my clients that the goal of an IMS is to make safety and environmental protection invisible—not meaning 'unseen,' but rather so deeply embedded in the quality process that you cannot produce the product without doing it safely and cleanly.
Pro Tip: When merging your systems, start with Internal Audits. Combine your audit teams so that a Quality Auditor learns to spot trip hazards (Safety) and an HSE Auditor learns to spot process deviations (Quality). This cross-pollination is the fastest way to mature your IMS culture.
Core Principles (The Shared DNA)
When I first started auditing over a decade ago, ISO standards were disjointed. ISO 9001 looked nothing like ISO 14001, and companies struggled to speak the same language across departments. That changed with the introduction of Annex SL (the High-Level Structure).
This shared DNA is the reason integration is possible today. It means that whether I am opening a Quality manual or an Environmental manual, Clause 9 is always "Performance Evaluation" and Clause 10 is always "Improvement." This structural alignment allows organizations to overlay their systems seamlessly, rather than building parallel bureaucracies.
The Plan-Do-Check-Act (PDCA) Cycle
Both QMS and EMS rely entirely on the PDCA cycle. This is the heartbeat of any management system. In the field, I often explain PDCA as the difference between "hoping for the best" and "managing for success."
Plan: Establish objectives based on risk. In QMS, this might be "reduce welding defects by 5%." In EMS, it translates to "reduce hazardous waste disposal by 10%."
Do: Implement the processes as planned. This is where the work happens—training, operation, and documentation.
Check: Monitor and measure. Are we hitting the 5% defect reduction? Is the waste actually decreasing?
Act: Take action to fix deviations or standardize success (Continual Improvement).
Risk-Based Thinking
Historically, many organizations treated safety and environment as reactive disciplines—fixing things only after they broke or leaked. Now, both ISO 9001:2015 and ISO 14001:2015 mandate "Risk-Based Thinking."
This is a favorite area for auditors to dig into. I expect to see a proactive approach:
In QMS: The risk might be a supply chain disruption causing a delivery delay, or a calibration error leading to product recall.
In EMS: The risk might be a change in legislation that bans a specific chemical byproduct, or a potential spill pathway near a storm drain.
Auditor Note: "I don't just look for a list of risks; I look for the link to action. If you identified a high risk of chemical spill in your register but have no spill kits or training in your 'Do' phase, that is a major non-conformance."
Leadership Commitment (Clause 5)
Neither system works if it is delegated solely to a junior officer or an external consultant. Clause 5 in both standards focuses heavily on "Leadership."
During an audit, I will interview the CEO or Site Manager, not just the Safety Manager. I ask them specifically how they ensure resources are available for both quality and environmental goals. If they cannot answer, the system is hollow.
Regulatory Emphasis: "Top management must demonstrate leadership and commitment... ensuring that the resources needed for the management system are available." — ISO High-Level Structure (Annex SL)
Pro Tip: When conducting your Management Review (Clause 9.3), do not hold separate meetings for Quality and Environment. Hold one "Business Systems Review." This forces leadership to see how a decision to buy cheaper raw materials (QMS risk) might increase waste disposal costs (EMS impact).
Implementation Roadmap: How to Get Started
Implementing these systems can often feel like climbing a mountain without a map. However, whether you are building a standalone QMS, an EMS, or an Integrated Management System (IMS), the journey follows a logical, step-by-step progression. In my experience guiding organizations through this process, success relies not on speed, but on a methodical approach.
Phase 1: Gap Analysis
Before writing a single policy or procedure, you must clearly understand your starting point. I always recommend conducting a formal "Gap Analysis" against the specific ISO standards (ISO 9001 or ISO 14001). This diagnostic phase prevents you from reinventing the wheel.
Identify Existing Controls: You likely already perform many required tasks. For example, you probably have waste disposal receipts (EMS evidence) or client delivery logs (QMS evidence).
Identify the "Missing Links": Pinpoint exactly where you fall short of the standard, such as lacking a formal internal audit procedure or a documented Context of the Organization.
Resource Assessment: Determine if your current team has the bandwidth to manage this, or if you need external support.
Phase 2: Documentation & Process Mapping
This is the phase where you define the "How." If you are aiming for an IMS, this is the moment to draft a single, unified Integrated Management Manual rather than two separate binders. This manual serves as the blueprint for your entire operation.
Define Context: clearly outline who you are, who your stakeholders are, and what external/internal issues affect you (Clause 4).
Draft SOPs: Write Standard Operating Procedures that reflect actual work, not theoretical ideals.
Map Processes: Visually map out how an order flows from customer inquiry to final delivery, highlighting quality checks and environmental impacts along the way.
Pro Tip: "Do not overwrite. Keep your procedures simple and accessible. If a procedure is too complex or bureaucratic, your workers will inevitably ignore it. As an auditor, if I see a complex procedure that isn't being followed, I am forced to issue a non-conformance finding. Compliance is easier when the rules are simple."
Phase 3: Training & Awareness
You cannot simply hand a worker a manual and expect compliance; you must train them. In my field audits, I interview frontline workers to ensure they understand why they are separating waste or measuring calipers, not just how to do it.
Toolbox Talks: specific, bite-sized training sessions focused on daily risks and quality requirements.
Competency Verification: It is not enough to just keep an attendance sheet. You must verify that the worker absorbed the training (e.g., through a test or practical demonstration).
Cultural Buy-in: Explain the personal benefits of the system—safety for them, stability for the company.
Phase 4: Internal Audit & Certification
Before you invite a prestigious external certification body—such as BSI, SGS, or TUV—you must stress-test your own system. An external audit should never be the first time your system is scrutinized.
Perform Internal Audits: Conduct a full cycle of audits covering every department. Be critical and honest about your findings.
Management Review: Hold a formal meeting where top leadership analyzes audit data, resource needs, and system performance.
The Certification Audit: Engage an external registrar for the Stage 1 (Documentation Review) and Stage 2 (Implementation Audit) process.
Real-World Applications & Case Examples
To ground this theory, let's look at how these concepts manifest in the field. I have audited diverse sectors—from heavy industry in the Gulf to corporate offices in Europe—and while the hazards change, the principles of Quality and Environment remain constant.
Manufacturing Sector
In a car manufacturing plant, the interaction between QMS and EMS is daily, critical, and often high-stakes. During an audit of an automotive assembly line, I look for two distinct streams of control:
Quality (The Product): I verify that the brake pads meet precise friction coefficients. If these components fail, the car does not stop, and lives are at risk. This is a direct ISO 9001 concern regarding product conformity and customer safety.
Environment (The Byproduct): Simultaneously, I inspect the painting booths. This process releases Volatile Organic Compounds (VOCs). An effective EMS ensures that air scrubbers are functional so the plant does not pollute the local air basin. If the scrubbers fail, the car is still "high quality," but the process is illegal.
Service Sector
There is a misconception that these standards only apply to factories. However, in the IT and consultancy sectors, QMS and EMS are increasingly vital for tendering major contracts.
Quality (The Service): In a software firm, QMS ensures that code is bug-free, delivered on time, and meets the client's scope of work. It is about process consistency and reducing "re-work."
Environment (The Footprint): Even without smokestacks, an IT firm has an impact. I look for how they manage electronic waste (e-waste)—are old servers and laptops recycled securely or dumped? Additionally, are they optimizing data center cooling to lower energy consumption?
Challenges and Expert Tips for Success
The road to certification is rarely smooth. In my experience, the biggest enemy of a good management system is not technical complexity, but self-inflicted bureaucracy.
Common Pitfall: "Paperwork Paralysis"
I often see organizations create systems that are designed to please an auditor rather than help the user. They produce thick binders of policies that sit on shelves, gathering dust until the day I arrive.
The "Fantasy Land" Problem: A common finding in my audit reports is a discrepancy between procedure and reality. If your written procedure states you inspect the forklift "daily," but the logs show you only do it "weekly," you have a non-conformance.
The Fix: Align the documentation with the actual practice. If a weekly inspection is safe and sufficient, change the procedure to say "weekly." Do not trap yourself with ambitious rules you cannot follow.
Expert Insight: "A management system must be a reflection of reality, not a wish list. As an auditor, I respect an honest system with minor gaps more than a 'perfect' paper system that nobody actually uses."
Cultural Buy-in
The hardest part of implementation is not the paperwork; it is the people. You can write the best SOP in the world, but if the technician on the night shift thinks it is a waste of time, the system will fail.
Shift the Mindset: You must move the culture from "we have to do this for the certificate" to "we do this because it makes our business better and safer."
Involve the Workforce: When writing procedures, involve the people who do the job. A machine operator knows the nuances of the equipment better than any external consultant. If they help write the rule, they will follow it.
Conclusion
Quality and Environmental Management Systems are two sides of the same coin: operational discipline. QMS ensures you are worthy of your customers' trust, and EMS ensures you are worthy of your community's trust.
In my years as an auditor, I have never seen a company with a strong EMS and QMS fail to improve its bottom line eventually. It reduces waste, prevents fines, and sharpens efficiency. But beyond the metrics and the certificates, there is a moral imperative. We have a duty to deliver safe products and to protect the world we live in. Implementing these systems is the first step in fulfilling that obligation.








Comments
Loading...